

Strong acids and bases are strong electrolytes, and weak acids and bases are weak COMMON ACIDS AND BASESĮlectrolytes.
Experimentally, the extent of ionization is determined by measuring the electrical conductance of solutions. Strong acids, such as hydrochloric acid, are 100 percent ionized in aqueous solution, whereas weak acids, such as acetic acid, are less than 5 percent ionized.

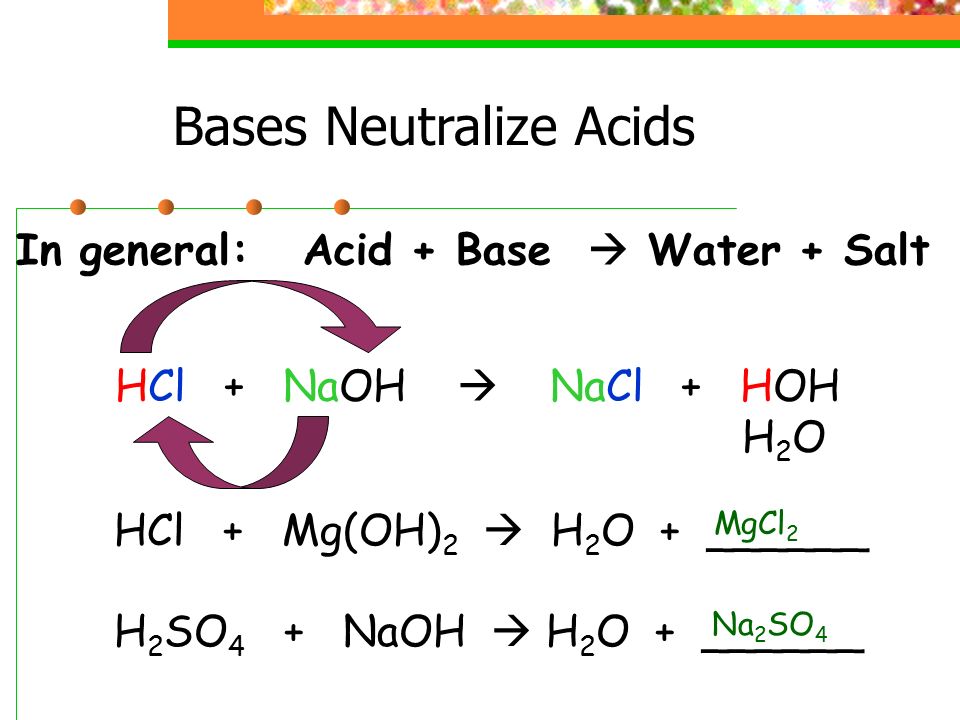
The strength of an acid or base is determined by the extent of its ionization in aqueous solution. H 3O + ( aq ) + OH − ( aq ) → 2 H 2O ( l ) (6) Strengths of Acids and Bases If H 3O + is substituted for H + ( aq ), the neutralization equation becomes This is referred to as the net ionic equation for the neutralization reaction. Ions common to both sides can be canceled to yield H + ( aq ) + Cl − ( aq ) K + ( aq ) + OH − ( aq ) → H 2O ( l ) K + ( aq ) + Cl − ( aq ) (4) Since HCl ( aq ) and KOH ( aq ) are fully ionized in solution, the preceding equation can be written as HCl ( aq ) + KOH ( aq ) → H 2O ( l ) + KCl ( aq ) (3) The second product is a salt, which is composed of the positive metal ion from the base and the negative ion from the acid. In the reaction of an acid with a base in aqueous solution, the hydrogen ions of the acid react with the hydroxide ions of the base to give water. It dissolves in water to give sodium ions and hydroxide ions. This water solution of HCl is referred to as hydrochloric acid.Ī typical base, according to the Arrhenius definition, is sodium hydroxide (NaOH). Hydrogen chloride (HCl), a gas, is an acid because it dissolves in water to yield hydrogen ions and chloride ions. Often, the hydronium ion or hydrated proton is represented as H + ( aq ). Later studies of aqueous solutions provided evidence of a small, positively charged hydrogen ion combining with a water molecule to form a hydrated proton, H +(H 2O) or H 3O +, which is called the hydronium ion. One of the earliest definitions of acids, advanced by the Swedish physicist and chemist Svante Arrhenius in 1887, stated that acid ionizes in aqueous solution to produce hydrogen ions (which are protons), H +, and anions and a base ionizes in aqueous solution to produce hydroxide ions (OH −) and cations. Most common acid-base reactions take place in water solutions (commonly referred to as aqueous solutions ). Hence, bases are considered the chemical opposite of acids. Bases change the colors of indicators (litmus turns from red to blue) and they neutralize acids. Besides having a sour taste, acids react with active metals to give hydrogen, they change the colors of indicators (for example, litmus turns from blue to red), and they neutralize bases. Vinegar is a 5 percent water solution of acetic acid. Another common acid is vinegar, which is the sour liquid produced when apple cider, grape juice, or other plant juices ferment beyond the formation of alcohol. Lemons, grapefruit, and limes taste sour because they contain citric acid and ascorbic acid ( vitamin C). The word "acid" comes from the Latin acidus, meaning "sour" or "tart," since water solutions of acids have a sour or tart taste. Acids and bases have been known by their properties since the early days of experimental chemistry.


 0 kommentar(er)
0 kommentar(er)
